Chair of Physical Chemistry
Research topics
Development of high temperature electrolysers
PhD Gunnar Nurk, PhD Ove Korjus, PhD Martin Maide et al

Harvesting of the wind and solar energy is possible by applying the complex PV and wind electricity generation combined with hydrogen electrolysis and storage steps. This is very important to keep the electricity flow in ac nets without very high current pulses. The hydrogen energy storage complex using pulsating electricity is 2-4 times cheaper than the batteries-based system. The batteries based system can be used as the short or medium-time energy storage technology due to the self-discharge phenomenon taking place in highly charged batteries. Institute of Chemistry, Chair of Physical Chemistry is developing together with the Estonian high-tech company H2Electro novel fully ceramic materials based high-temperature water electrolysis systems. Energy-efficient high-temperature electrolysis complexes are inevitable for the realization of the Green Deal and other ambitious projects for the modernization of the European high-technology industry. Electrolysis of water at room temperature is an old method but it is very energy-consuming in comparison with high-temperature electrolysis, where the residual heat can be used for activation of the water molecules reduction
.Application of hydrogen technologies is mainly held back by the moderate price of hydrogen generated by low-temperature electrolysis systems. However, molecular hydrogen can be used as the green-sustainable fuel for different types of vehicles starting from small cars, vans, trains, trucks, ships, and ferry-boats, including airplanes and rockets: As a reducing agent for the production of iron(soft Fe free from carbon contaminants, high melting temperature powder metals, aluminum, and clean rare earth metals, ammonia for the chemical industry and fertilizers, as a fuel for fuel cells for co-generation of electricity and heat. The complex solid oxide materials can be used as CO2 and H2O co-electroreduction devices for the generation of so-called syngas applicable for the production of very many chemicals including alcohols, esters, aldehydes, carboxylic acids, polycarbonates, different organic solvents like ethylene carbonate, propylene carbonate, etc., applicable in Na-ion batteries and supercapacitors. In addition, the complex oxide materials can be used for electroreduction of nitrogen oxides and sulfur oxides, thus cleaning the air from fossil fuel-generated contaminants. New complex oxides are under intensive development for novel electrolysis systems at the University of Tartu, Institute of Chemistry.
Development of solid oxide fuel cells and polymer membrane fuel cells
PhD Gunnar Nurk, PhD Indrek Kivi, PhD Jaak Nerut, PhD Rutha Jäger, prof. Enn Lust et al

One very important development direction of sustainable energy technologies is based on the application of electrochemical fuel conversion devices to electricity and heat, called the fuel cells. At the moment the two types of fuel cells are under intensive development at the University of Tartu. The moderate temperature polymer electrolyte fuel cells are working at 60-80 oC and clean hydrogen is used as a fuel to co-generate the electricity and heat. These devices are based on the nano-structural catalysts activated with Pt- metal nanoparticles or Fe- nitrogen, Co-nitrogen as well as Fe-Co-nitrogen binary catalysts. The main problem is the high price of Pt-metals-based complex nanostructural electrodes and low durability and cyclability under high current pulses conditions. In addition, there is a very limited amount of Pt metals under mining and production. Taking into account that the preparation of PEMFc is comparatively simple the PEM FC system has been installed in 2021 to Iseauto (self-driving car) according to the contract with Estonian company AuveTech OY.
The solid oxide fuel cells are based on complex perovskite mixture conducting oxides and d-metal (Ni-) cermet anodes and fully ionic conducting ceria or zirconia, activated with Gd or Y, electrolytes ( or multilayers based electrolytes) working at higher temperatures giving higher energy efficiency for the generation of electricity. The currents applicable in SOFC are higher and the pulsating high current density pulses do not have so destructive influence as similar current pulses if applied at moderate temperatures. The solid oxide fuel cells production methods worked out at UT from 2001 to 207 have been patented and now Elcogen AS is the leading SOFC producing company in the EU. Elcogen AS/OY won the main innovation price at 2019, and the Estonian innovation prize at 2020. Differently from PEM FC the different hydrogen-containing organic compounds can be used as fuels including methane, methanol, ethanol, esters, ethers, cyclic organic compounds as well as natural gas, gasoline, and diesel. At the moment very intensive studies to replace the Ni-cerment and other D-metal cermet anodes with fully ceramic materials are under very intensive studies at UT IC.
Development of energy harvesting demo-center and FC powered self-driving car
Rait Kanarbik, Peeter Valk, PhD Jaak Nerut, prof. Enn Lust et al

One of the development directions of the institute of chemistry was/is to develop the photovoltaic (PV) energy harvesting demo-center for real testing of modern energy collecting, storage and regeneration devices. Sustainable energy demo-center consists of:60kW PV panels, Pb/PbO2 batteries(24 pieces 108Ah each), 6.3 kW alkaline electrolyser, 300 bar hydrogen storage tanks (12 pieces) and finally different type of fuel cells applied for regeneration of electricity and heat. During day-time the additional electricity (not used for generation electricity during day-time due to the limited power of electrolyser) storaged in batteries is used for production of hydrogen during nights or cloudy days. Our electrolyser is producing 2kg hydrogen per day. The storage hydrogen is used for conducting the fundamental research and applied studies using PEMFC or solid oxide fuel cells as well for feeding of fuel for the PEM FC powered self-driving car IseAuto. Based on the contract with AuveTech OÜ, UT IC completed in 2020-2021 the first self-driving car in World. In addition UTIC completed the PEM FC powered coffee machine and pancake producing machines to demonstrate how the hydrogen technology can be used in places, where there is no electrical grids, or if there is electrical blackout.
Taking into consideration that the small PV field combined with electricity storage/ regeneration application possibilities is very energy and cost effective , as well as account the Estonian electricity prices, the widening of PV field up to 120-140 kW is under planning and projecting stage.
Fundamental in situ interfacial studies
PhD Piret Pikma, PhD Liis Siinor, PhD Vladislav Ivaništšev, MSc Heigo Ers
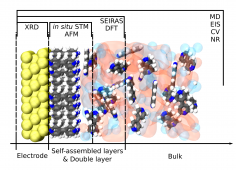
The fundamental understanding of the occurring processes needs to be with high scientific excellence and in accordance with the latest discoveries to develop new techniques and devices. However, more complex real-life applications are still constrained by the lack of knowledge in the level of first principles. For the reconciliation of the gap between the theory and application knowledge of the interfacial effects need to be gathered. This is achieved by studying different aspects of numerous electrochemical model systems to unveil the relations between different components, such as electrode’s crystal structure and electronic properties, adsorbate geometric and chemical qualities, the impact of ionic liquids and other electrolytes interfacial properties as well as the applied potential.
For gaining a holistic picture our group utilizes different in situ experimental (e.g. in situ scanning tunnelling and atomic force microscopy) as well as computational (e.g. density functional theory and molecular dynamics) techniques to study the electrode | electrolyte interfacial properties.



